![]()
Unlock Academic Success with
Kelevo School of Growth
Division of Kelevo Academy!
At Kelevo School of Growth (KSG), we offer expert coaching for Classes 9 to 12 in all major school subjects, empowering students with personalized guidance, interactive sessions, and focused exam preparation.
- Highly Qualified Teachers
- Concept-based Learning
- Regular Assessments & Doubt Clearing
- NCERT-aligned Curriculum
- Proven Results & Academic Excellence
Our Courses
We are delighted to introduce a variety of dynamic courses packed with innovative features designed to enhance your learning experience.
Our Teachers
“We have some of the best and most dedicated teachers in town ready to help you unlock your full potential and refine your skills to perfection.”
Admission Proccess
We are excited to announce that admissions for our new batch are now open! Seats are limited, so don’t delay—secure your spot today and start your journey to success.
Explore Your Courses
Meet Our Expert Teachers
Explore Now


ABOUT US
Welcome to Kelevo School of Growth – A Division of Kelevo Academy!
At Kelevo School of Growth (KSG), we specialize in delivering high-quality coaching for Classes 9 to 12 across all major subjects, combining academic rigor with personalized support to help students excel. Our programs are thoughtfully designed to foster academic excellence and holistic development through innovative teaching methods, expert faculty guidance, and a strong focus on concept clarity. Students benefit from:
- Expert Faculty
- Personalized Attention
- Instant Doubt Resolution
- Regular Assessments
- Comprehensive NCERT-Aligned Study Material
- One-on-One Academic Mentorship
Classes
Find Best Classes For Yourself!
Class 9
Class 10
Class 11
Class 12
POPULAR COURSES
OUR COURSES
 FreeClass 9
FreeClass 9
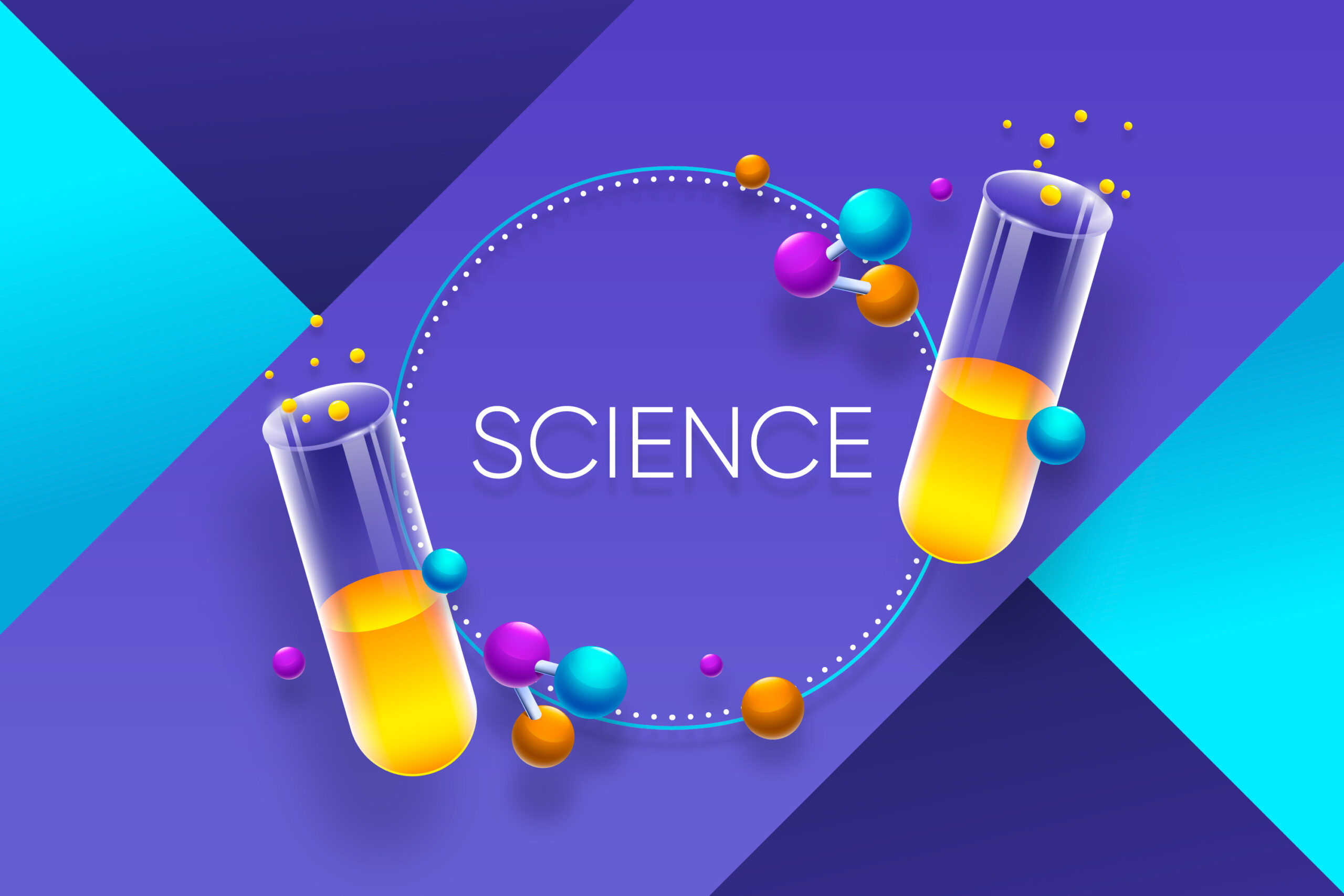
Science
Course OverviewThis course introduces students to fundamental scientific concepts across Physics, Chemistry, and Biology. It emphasizes understanding principles, developing analytical…
- 0 Lessons
- 0 Students
 FreeClass 9
FreeClass 9
Mathematics
Course OverviewThis course introduces students to fundamental mathematical concepts, laying the groundwork for higher-level studies. It emphasizes understanding and application…
- 0 Lessons
- 0 Students
 FreeClass 10
FreeClass 10
Science
Course OverviewThis course builds upon the foundational knowledge from Class 9, delving deeper into scientific concepts and their applications. It…
- 0 Lessons
- 0 Students
 FreeClass 10
FreeClass 10
Mathematics
Course OverviewThis course builds upon the foundations laid in Class 9, introducing more complex concepts and applications, preparing students for…
- 0 Lessons
- 0 Students
More Courses
TESTIMONIALS
What Our Students Have To Say
Hear directly from the learners who’ve experienced the Kelevo School of Growth difference
“Maths used to be my weakest subject, but everything changed after joining this class. The way concepts are explained with real-life examples and step-by-step methods made it so much easier to understand. “
Riya Sharma
Class 9
“Being a PCM student in Class 12 was both challenging and rewarding. The syllabus was vast, especially with subjects like Physics and Mathematics, but the right guidance and consistent practice made a huge difference. “
Ashish Mishra
Class 12 PCM
“Kelovo Academy has truly transformed the way I learn. In Class 10, the pressure of board exams can be overwhelming, but the teachers here made everything so clear and manageable.”
Aarav Mehta
Class 10 Student
“Joining Kelovo Academy was the best decision I made for my Class 12 PCM preparation. The faculty here is outstanding—always ready to clarify doubts, and they explain even the toughest concepts in a simple way. The regular tests, personalized feedback, and revision sessions helped me improve my performance consistently.”
Ritika Sharma
Class 12 (PCM)



Get In Touch:
info@kelevoacademy.com
or
Call Us Via:
+91 70110 73638

ITS EASY, ITS BRILLIANT, IT WORKS!
“To reach new heights, one must begin the climb—and when the journey gets tough, perseverance becomes the key to success.”
Over the years, I’ve learned that success in academics isn’t just about hard work—it’s about focused effort and the right guidance. Knowing what to study, and equally important, what not to, can make all the difference in a student’s academic journey.
At Kelevo Academy, our innovative teaching methodologies and student-centric approach have consistently helped thousands of learners turn their aspirations into achievements. As exam patterns evolve, we continuously adapt to ensure our students receive the best support possible. We embrace the new NECT curriculum with confidence and commitment, ready to guide our students towards excellence.
Rakhi Chauhan
M.Com, Chartered Accountant
Director Message
OUR PARTNERS
Learn with Our Partners


LATEST ARTICLES
Get News with Kelevo-Academy
Crafting Effective Learning Guide Line
- 15 Nov, 2023
- Com 0
aConsectetur adipisicing elit, sed do eiusmod tempor inc…
Exploring Learning Landscapes in Academic
- 14 Nov, 2023
- Com 3
Consectetur adipisicing elit, sed do eiusmod tempor inc idid unt ut labore et dolore magna aliqua enim ad…
Voices from the Learning Education Hub
- 13 Nov, 2023
- Com 0
Consectetur adipisicing elit, sed do eiusmod tempor inc idid unt ut labore…